CPAP Recall and Alternative Treatment Options

On June 14, 2021, Philips issued a recall notification for the United States for specific affected ventilation and sleep apnea devices. The notification informs patients, users and customers of potential impacts on patient health and clinical...
On June 14, 2021, Philips issued a recall notification for the United States for specific affected ventilation and sleep apnea devices. The notification informs patients, users and customers of potential impacts on patient health and clinical use related to this issue.
Over 3.5 million Philips Respironics CPAP and BiPAP machines sold between 2009 and April 2021 are recalled, due to toxic foam used in the breathing devices.
RECOMMENDATIONS FOR BIPAP OR CPAP USERS
Philips advises that patients using recalled BPAP and CPAP devices should immediately consult with their medical providers to determine the most appropriate options for continued treatment, based on the benefits of continuing therapy and potential identified risks.
The ultimate judgment regarding any specific care must be made by the treating clinician and the patient, taking into consideration the individual circumstances of the patient, available treatment options, and resources.
Philips claims these toxic particles may cause permanent and even life-threatening issues. According to the US Food and Drug Administration (FDA), the polyester-based polyurethane (PE-PUR) sound abatement foam, which is used to reduce sound and vibration in these affected devices, may break down and potentially enter the device’s air pathway. If this occurs, black debris from the foam or certain chemicals released into the device’s air pathway may be inhaled or swallowed by the person using the device.
The potential health consequences of this problem are serious. Philips has identified the following to be among the potential health risks of using a recalled device:
- Airway inflammation
- Skin, eye, and respiratory tract irritation (including upper airway irritation)
- Headache
- Asthma
- Toxic carcinogenic effects
- Cough
- Chest pressure
- Sinus infection
The following Philips Respironics devices that were manufactured between 2009 and April 26, 2021 are included in the recall.

(For details, see Philips’ Respironics recall notificationExternal Link Disclaimer (PDF).)
REPLACEMENT RECOMMENDATIONS FOR BIPAP OR CPAP USERS
Philips is committed to rectifying this issue through a robust and comprehensive program. They have established a claims processing and support center to assist their customers, but many are finding it difficult to find replacement units.
Patients have reported that calls to the toll-free phone number provided do not connect callers with people knowledgeable about the recall, and requests for return phone calls are not fulfilled. This has led many people with obstructive sleep apnea to resort to other options and research alternative treatments.
WHAT ARE SOME OTHER ALTERNATIVES TO CPAP?
For people who suffer from Obstructive Sleep Apnea, there may be an alternative treatment method, depending on the severity of your apnea. Here are some options that you may want to discuss with your healthcare provider:
- Oral Appliances. Oral appliances such as mouthguards can help hold the tongue in place or ease the jaw forward, helping to keep the airway free and open.
- Oral Surgery. In some cases, genetics can be the cause of sleep apnea. People born with big tonsils or extra tissue in their throats may have trouble breathing while they sleep. Other issues may involve the tongue, jaw, or soft palate. Oral surgeons can work with a patient to make permanent changes to their anatomy and help them breathe easier.
- Weight Loss. One of the major risk factors for having sleep apnea is obesity. Studies show that shedding just 10 percent of body weight can help alleviate sleep apnea symptoms. Dropping more pounds can even cure sleep apnea in some cases.
- Positional Therapy. If you sleep on your stomach or your side, you’re less likely to experience sleep apnea. If you sleep on your back, though, you’re more likely to experience problems. Devices that attach to your waist or back can help keep you from lying on your back and reduce the need for a CPAP machine.
- Inspire Therapy. For patients who’ve tried and failed to treat their sleep apnea with all kinds of things, the Inspire implant can be an option. The battery-operated device is surgically planted into the chest and, when turned on at bedtime, can function for eight hours.
WHY IS ORAL APPLIANCE THERAPY AN IDEAL REPLACEMENT OPTION?
An
oral appliance is a device that works in your mouth during sleep. It looks a lot like an orthodontic retainer or a sports mouth guard, although the technology behind oral appliances is more advanced.
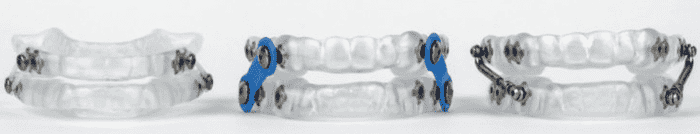
Oral appliances are available from many dental offices, but most prefer to select one type of oral appliance and offer it to all patients. Not everyone has the same set of needs (or the same anatomy) so there is a range of options to find the right fit for your needs.
It’s important to understand that finding the right oral appliance for you is only the first step. You must also incorporate using your oral appliance into your bedtime routine to reap the benefits of this form of therapy.
HOW MUCH DO ORAL APPLIANCES COST?
One of the most common questions we get asked at Pennsylvania Dental Sleep Medicine (PDSM) is how much it costs to be treated with an oral appliance. Unfortunately, there is no cut-and-dry answer, but most major insurances will cover it the same way they do with CPAP. If you have met your deductible, it can be little to no out-of-pocket cost.
So, if CPAP is not an option right now, or you prefer to try a different approach, Pennsylvania Dental Sleep Medicine offers oral appliances as a great alternative to CPAP.
You can schedule an appointment online or by phone today at (717) 995-3590 to explore treatment options. The team of sleep dentists has guided more than 18,000 patients through sleep apnea treatment, and each team member has achieved diplomate status through the American Board of Dental Sleep Medicine.


